Abstract
Objective: Cellular therapies, including CAR-T cell, gene therapy, autologous and allogeneic hematopoietic cell transplantation (HCT), remain a cornerstone in management of various benign and malignant disorders. Despite intensified efforts to improve strategies, invasive fungal infections (IFD) remain an important cause of morbidity and mortality. Therefore, we investigated risk factors, prevalence, and outcome of IFD in the era of novel anti-fungal prophylaxis.
Methods: We retrospectively enrolled consecutive adult patients that underwent cellular therapies at our JACIE-accredited center according to standard operating procedures (2013-2022). We defined IFD according to current consensus (2020) and guidance on imaging (2021, EORTC). We analyzed the following factors: age, disease, donor, HLA matching, conditioning, graft, severe acute and moderate/severe chronic GVHD, antifungal prophylaxis, immunosuppressants, IFD type (according to current consensus), galactomannan/β-D-glucan, cultures, imaging, bacterial/viral infections, IFD treatment, relapse, and overall survival (OS).
Results: We studied 950 recipients of cellular therapies (19 CAR-T cell and 2 gene therapies, 456 autologous and 473 allogeneic HCT). No CAR-T cell and gene therapy patient developed IFD; while 3/456 autologous HCT recipients who suffered from primary refractory/relapsed lymphomas presented with probable IFD. Further analysis was performed only in allogeneic HCT recipients.
Possible IFD was documented in 31/473 at a median of 112 (range 7-1353) post-transplant day, probable in 11/473 (5: positive galactomannan, 6: positive cultures) at 154 (12-469), proven in 10/473 (3: positive galactomannan, 7: positive cultures) at 226 (25-1146) day. Concurrence of bacterial or viral infection was documented in 20/52 and 17/52 patients respectively. Second IFD episode occurred in 3 patients (1: probable, 2: proven IFD), that succumbed to TRM (1) and relapse (2). Candida species were isolated in 9 patients, Fusarium in 2 and Aspergillus in 2. Mucormycosis was diagnosed in 2 patients, while the remaining IFD were attributed to aspergillosis. During the aplastic period, caspofungin had been administered as primary prophylaxis (41/52) and amphotericin as secondary prophylaxis (11/52), which were then changed to posaconazole and isavuconazole respectively for the immunosuppression period.
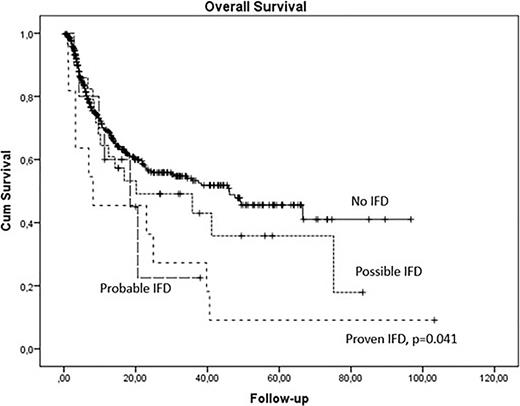
Independent risk factors for IFD were type of donor (20% in alternative, 12% in unrelated, and 5% in sibling donors, p=0.006) and moderate/severe chronic GVHD (15% versus 8%, p=0.029). Five-year OS was significantly lower in patients with IFD, especially those with proven IFD (p=0.041). IFD remained a predictor of OS (p=0.044) independently of donor type and chronic GVHD.
Conclusions: Our large real-world cohort indicates the relatively low prevalence of proven IFD in the era of novel antifungal prophylaxis, which is however associated with poor outcomes in allogeneic HCT recipients. Possible IFD, assessed by host and clinical factors including updated documentation of imaging, has shown similar outcomes to probable IFD. Therefore, wider application of sensitive mycological testing is needed for high-risk populations, such as alternative alloHCT recipients or chronic GVHD patients.
Disclosures
Gavriilaki:Jazz: Research Funding; Sanofi: Honoraria; Omeros: Honoraria; Alexion: Honoraria; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal